Researchers show that memories reside in specific brain cells
March 22, 2012
MIT News
Simply activating a tiny number of neurons can conjure an entire memory.
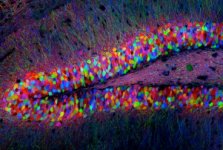
An image of a transgenic mouse hippocampus.
Our fond or fearful memories — that first kiss or a bump in the night — leave memory traces that we may conjure up in the remembrance of things past, complete with time, place and all the sensations of the experience. Neuroscientists call these traces memory engrams.
But are engrams conceptual, or are they a physical network of neurons in the brain? In a new MIT study, researchers used optogenetics to show that memories really do reside in very specific brain cells, and that simply activating a tiny fraction of brain cells can recall an entire memory — explaining, for example, how Marcel Proust could recapitulate his childhood from the aroma of a once-beloved madeleine cookie.
“We demonstrate that behavior based on high-level cognition, such as the expression of a specific memory, can be generated in a mammal by highly specific physical activation of a specific small subpopulation of brain cells, in this case by light,” says Susumu Tonegawa, the Picower Professor of Biology and Neuroscience at MIT and lead author of the study reported online today in the journal Nature. “This is the rigorously designed 21st-century test of Canadian neurosurgeon Wilder Penfield’s early-1900s accidental observation suggesting that mind is based on matter.”
In that famous surgery, Penfield treated epilepsy patients by scooping out parts of the brain where seizures originated. To ensure that he destroyed only the problematic neurons, Penfield stimulated the brain with tiny jolts of electricity while patients, who were under local anesthesia, reported what they were experiencing. Remarkably, some vividly recalled entire complex events when Penfield stimulated just a few neurons in the hippocampus, a region now considered essential to the formation and recall of episodic memories.
Scientists have continued to explore that phenomenon but, until now, it has never been proven that the direct reactivation of the hippocampus was sufficient to cause memory recall.
Shedding light on the matter
Fast forward to the introduction, seven years ago, of optogenetics, which can stimulate neurons that are genetically modified to express light-activated proteins. “We thought we could use this new technology to directly test the hypothesis about memory encoding and storage in a mimicry experiment,” says co-author Xu Liu, a postdoc in Tonegawa’s lab.
“We wanted to artificially activate a memory without the usual required sensory experience, which provides experimental evidence that even ephemeral phenomena, such as personal memories, reside in the physical machinery of the brain,” adds co-author Steve Ramirez, a graduate student in Tonegawa’s lab.
The researchers first identified a specific set of brain cells in the hippocampus that were active only when a mouse was learning about a new environment. They determined which genes were activated in those cells, and coupled them with the gene for channelrhodopsin-2 (ChR2), a light-activated protein used in optogenetics.
Next, they studied mice with this genetic couplet in the cells of the dentate gyrus of the hippocampus, using tiny optical fibers to deliver pulses of light to the neurons. The light-activated protein would only be expressed in the neurons involved in experiential learning — an ingenious way to allow for labeling of the physical network of neurons associated with a specific memory engram for a specific experience.
Finally, the mice entered an environment and, after a few minutes of exploration, received a mild foot shock, learning to fear the particular environment in which the shock occurred. The brain cells activated during this fear conditioning became tagged with ChR2. Later, when exposed to triggering pulses of light in a completely different environment, the neurons involved in the fear memory switched on — and the mice quickly entered a defensive, immobile crouch.
False memory
This light-induced freezing suggested that the animals were actually recalling the memory of being shocked. The mice apparently perceived this replay of a fearful memory — but the memory was artificially reactivated. “Our results show that memories really do reside in very specific brain cells,” Liu says, “and simply by reactivating these cells by physical means, such as light, an entire memory can be recalled.”
Referring to the 17th-century French philosopher who wrote, “I think, therefore I am,” Tonegawa says, “Ren? Descartes didn’t believe the mind can be studied as a natural science. He was wrong. This experimental method is the ultimate way of demonstrating that mind, like memory recall, is based on changes in matter.”
“This remarkable work exhibits the power of combining the latest technologies to attack one of neurobiology’s central problems,” says Charles Stevens, a professor in the Molecular Neurobiology Laboratory at the Salk Institute who was not involved in this research. “Showing that the reactivation of those nerve cells that were active during learning can reproduce the learned behavior is surely a milestone.”
The method may also have applications in the study of neurodegenerative and neuropsychiatric disorders. “The more we know about the moving pieces that make up our brains,” Ramirez says, “the better equipped we are to figure out what happens when brain pieces break down.”
Other contributors to this study were Karl Deisseroth of Stanford University, whose lab developed optogenetics, and Petti T. Pang, Corey B. Puryear and Arvind Govindarajan of the RIKEN-MIT Center for Neural Circuit Genetics at the Picower Institute for Learning and Memory at MIT. The work was supported by the National Institutes of Health and the RIKEN Brain Science Institute.
March 22, 2012
MIT News
Simply activating a tiny number of neurons can conjure an entire memory.

An image of a transgenic mouse hippocampus.
Our fond or fearful memories — that first kiss or a bump in the night — leave memory traces that we may conjure up in the remembrance of things past, complete with time, place and all the sensations of the experience. Neuroscientists call these traces memory engrams.
But are engrams conceptual, or are they a physical network of neurons in the brain? In a new MIT study, researchers used optogenetics to show that memories really do reside in very specific brain cells, and that simply activating a tiny fraction of brain cells can recall an entire memory — explaining, for example, how Marcel Proust could recapitulate his childhood from the aroma of a once-beloved madeleine cookie.
“We demonstrate that behavior based on high-level cognition, such as the expression of a specific memory, can be generated in a mammal by highly specific physical activation of a specific small subpopulation of brain cells, in this case by light,” says Susumu Tonegawa, the Picower Professor of Biology and Neuroscience at MIT and lead author of the study reported online today in the journal Nature. “This is the rigorously designed 21st-century test of Canadian neurosurgeon Wilder Penfield’s early-1900s accidental observation suggesting that mind is based on matter.”
In that famous surgery, Penfield treated epilepsy patients by scooping out parts of the brain where seizures originated. To ensure that he destroyed only the problematic neurons, Penfield stimulated the brain with tiny jolts of electricity while patients, who were under local anesthesia, reported what they were experiencing. Remarkably, some vividly recalled entire complex events when Penfield stimulated just a few neurons in the hippocampus, a region now considered essential to the formation and recall of episodic memories.
Scientists have continued to explore that phenomenon but, until now, it has never been proven that the direct reactivation of the hippocampus was sufficient to cause memory recall.
Shedding light on the matter
Fast forward to the introduction, seven years ago, of optogenetics, which can stimulate neurons that are genetically modified to express light-activated proteins. “We thought we could use this new technology to directly test the hypothesis about memory encoding and storage in a mimicry experiment,” says co-author Xu Liu, a postdoc in Tonegawa’s lab.
“We wanted to artificially activate a memory without the usual required sensory experience, which provides experimental evidence that even ephemeral phenomena, such as personal memories, reside in the physical machinery of the brain,” adds co-author Steve Ramirez, a graduate student in Tonegawa’s lab.
The researchers first identified a specific set of brain cells in the hippocampus that were active only when a mouse was learning about a new environment. They determined which genes were activated in those cells, and coupled them with the gene for channelrhodopsin-2 (ChR2), a light-activated protein used in optogenetics.
Next, they studied mice with this genetic couplet in the cells of the dentate gyrus of the hippocampus, using tiny optical fibers to deliver pulses of light to the neurons. The light-activated protein would only be expressed in the neurons involved in experiential learning — an ingenious way to allow for labeling of the physical network of neurons associated with a specific memory engram for a specific experience.
Finally, the mice entered an environment and, after a few minutes of exploration, received a mild foot shock, learning to fear the particular environment in which the shock occurred. The brain cells activated during this fear conditioning became tagged with ChR2. Later, when exposed to triggering pulses of light in a completely different environment, the neurons involved in the fear memory switched on — and the mice quickly entered a defensive, immobile crouch.
False memory
This light-induced freezing suggested that the animals were actually recalling the memory of being shocked. The mice apparently perceived this replay of a fearful memory — but the memory was artificially reactivated. “Our results show that memories really do reside in very specific brain cells,” Liu says, “and simply by reactivating these cells by physical means, such as light, an entire memory can be recalled.”
Referring to the 17th-century French philosopher who wrote, “I think, therefore I am,” Tonegawa says, “Ren? Descartes didn’t believe the mind can be studied as a natural science. He was wrong. This experimental method is the ultimate way of demonstrating that mind, like memory recall, is based on changes in matter.”
“This remarkable work exhibits the power of combining the latest technologies to attack one of neurobiology’s central problems,” says Charles Stevens, a professor in the Molecular Neurobiology Laboratory at the Salk Institute who was not involved in this research. “Showing that the reactivation of those nerve cells that were active during learning can reproduce the learned behavior is surely a milestone.”
The method may also have applications in the study of neurodegenerative and neuropsychiatric disorders. “The more we know about the moving pieces that make up our brains,” Ramirez says, “the better equipped we are to figure out what happens when brain pieces break down.”
Other contributors to this study were Karl Deisseroth of Stanford University, whose lab developed optogenetics, and Petti T. Pang, Corey B. Puryear and Arvind Govindarajan of the RIKEN-MIT Center for Neural Circuit Genetics at the Picower Institute for Learning and Memory at MIT. The work was supported by the National Institutes of Health and the RIKEN Brain Science Institute.
