David Baxter PhD
Late Founder
A guide to switching antidepressant therapy
Jan 1, 2007
By KEVIN SCOTT FERENTZ, MD
Patients with depression who previously did not achieve remission with antidepressant drug therapy can be helped with an additional medication or a different antidepressant according to the STAR*D study. By learning how to switch from one drug to another, you can help more patients attain complete relief from their depression.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study released in 2006, the nation's largest depression study, funded by the National Institutes of Health's National Institute of Mental Health (NIMH), shows that people whose depression is resistant to initial treatment can achieve remission and symptom relief when treated with a secondary strategy that either augments or switches medications. STAR*D is the first study to examine the effectiveness of different treatment strategies for those who did not become symptom free after initial medication.1
An overall assessment of the study suggests that a patient with persistent depression can get well after trying several treatment strategies, but his or her odds of beating the depression diminish as additional treatment strategies are needed.1
The goal of the treatment of depression is remission, meaning the complete or near-complete resolution of all symptoms.2 Current guidelines suggest the acute phase of treatment?when medication is started?generally lasts for up to 12 weeks. In the acute phase, the treatment goal is to get the patient into remission, the point at which symptoms are completely, or nearly completely, gone. Once patients are in remission, they enter the maintenance phase of treatment, which should last for an additional 4 to 9 months.3 The problem is that many patients do not remain on medication long enough to reach remission, let alone stay on medication for the additional recommended months. Up to 33% of patients stop medication within the first month, and up to 44% discontinue medication within 3 months of initiating therapy.4-6
In the STAR*D trial, 3600 adults with nonpsychotic major depressive disorder received 1 to 4 successive acute treatment steps. Those who achieved symptom remission could enter a 12-month naturalistic follow-up phase. Remission was defined as a score of 5 or less on the Quick Inventory of Depressive Symptomatology-Self-Report or a score of 7 or less on the Hamilton Rating Scale for Depression (HAM-D).1 Remission rates in the STAR*D trial were 37%, 31%, 14%, and 13% for the first, second, third, and fourth acute treatment steps, respectively. In step 1, subjects received citalopram (Celexa) as their first treatment step. Step 2 provided 7 possible treatments involving 4 switch treatments?citalopram was stopped and new treatment initiated with sustained-release bupropion (Wellbutrin), cognitive therapy, sertraline (Zoloft), or extended-release venlafaxine (Effexor) and 3 augmentation options (citalopram plus bupropion, buspirone [BuSpar], or cognitive therapy). Step 3 included 2 medication switch strategies (mirtazapine [Remeron] or nortriptyline [Pamelor]) or 2 medication augmentation strategies (lithium or T3). Level 3 entailed randomization to either tranylcypromine (Parnate) or extended-release venlafaxine. The investigators conclude that lower acute remission rates and higher relapse rates can be expected when more treatment steps are needed.1
Two of the many reasons patients discontinue antidepressant medication are intolerance to the medication (adverse events) and lack of response (either partial or complete). When a patient is having intolerable side effects, or when a patient is not responding to an antidepressant, clinicians often choose to switch patients from one antidepressant to another.
SIDE EFFECTS OF ANTIDEPRESSANTS
Side effects from antidepressant medication include those that occur during the acute phase of treatment, as well as those that become problematic during long-term treatment, such as weight gain and sexual dysfunction. Consequently, comparing side-effect profiles of different antidepressants often requires comparing combinations of different clinical trials, all of which use different methods of reporting side effects. As a result, it is difficult to get a true picture of the adverse effects one can expect from any single antidepressant in an individual patient. Many beliefs about the side-effect profiles of antidepressants are based on the marketing data of pharmaceutical companies and our own personal experiences with small numbers of patients.
MANAGING COMMON SIDE EFFECTS
Generally speaking, the adverse effects that are most likely to necessitate a change in medication occur early in treatment; the exceptions are the late-onset side effects of weight gain and sexual dysfunction. The most common early adverse effects of selective serotonin reuptake inhibitors (SSRIs) are nausea and headache. But these effects are usually relatively mild, and usually resolve within 2 to 4 weeks.7 Taking medications with meals or at bedtime often helps minimize these side effects. The SSRIs, as a class, are generally sedating, with paroxetine (Paxil), the most anticholinergic, often considered the most sedating of the group. Mirtazapine and trazodone (Desyrel) are also highly sedating, while nefazodone is somewhat less so.
If patients find that their medication has a sedative effect, they should be told to take it at bedtime. Bupropion is the only antidepressant that can cause insomnia in more than a few patients. Even though this effect is seen much less often with the longer-acting formulations, because of its potential activating effect, bupropion should generally be taken in the morning. The side-effect profile of venlafaxine, a serotonin and norepinephrine reuptake inhibitor, is much like that of the SSRIs. At higher doses, however, it can cause hypertension. This is believed to be due to the enhanced reuptake inhibition of norepinephrine at doses of 150 mg and higher. Approximately 3% of patients treated with 75 to 375 mg/d of sustained-release venlafaxine experience nontransient BP.8 Duloxetine (Cymbalta), a recently released dual reuptake inhibitor, appears less likely to cause hypertension.
Up to 60% of patients taking an SSRI will experience some degree of sexual dysfunction.7 This may manifest as delayed orgasm, inability to have an orgasm, and/or decreased libido. Manufacturers may claim different rates of sexual dysfunction for their various products, but this adverse effect appears to be a class phenomenon. A large multicenter trial based in primary care practices revealed that all SSRIs, venlafaxine, and mirtazapine caused similar rates of sexual dysfunction, while bupropion and nefazodone were least likely to cause sexual dysfunction.9 A recent review details the various pharmacologic strategies for dealing with this common problem.10 Weight gain?averaging 3 to 4 kg?is also a common late side effect of antidepressants. Nutritional counseling and exercise are usually the only measures necessary to deal with this problem. Mirtazapine is associated with larger amounts of weight gain.
PARTIAL AND NO RESPONSE
In most cases, patients placed on a reasonable dosage of an antidepressant should show some response after 4 weeks. If patients have shown no response after 3 to 4 weeks on an antidepressant, there is less than a 20% chance they will show a clinical response after 6 to 8 weeks.11 Overall, approximately 29% to 46% of depressed patients will show only partial or no response to antidepressant treatment.12 A more specific estimate is that 12% to 15% of patients will partially respond and 19% to 34% will not respond at all to a given antidepressant medication.12
Options available to patients who do not achieve remission on a single antidepressant include dosage increase, augmentation with a second drug, and switching to a new medication. In a survey of 432 attendees (mostly psychiatrists) at a psychopharmacology course, clinicians were asked what they would do when faced with a patient who either partially responded or did not respond to 8 weeks of treatment with an SSRI.13 The most common first-choice treatment strategy for partial responders was to raise the dosage of the SSRI. For nonresponders, the most popular choice was to switch to a non-SSRI antidepressant. There are data that indicate switching from one SSRI to another may be beneficial if a patient is either intolerant to or not responding to one particular SSRI.14,15 There is also evidence that suggests that it can be useful to switch to a non-SSRI when faced with a nonresponder.
The issue of augmentation versus switching is beyond the scope of this article but was recently addressed by a large expert panel.16 The panel concluded that "simultaneous targeting of both the noradrenergic and serotonergic systems is one of the most effective augmentation strategies." The panel also recommended switching to an agent of a different class in patients who fail to respond to first-line therapy.
HOW TO KEEP PATIENTS ON MEDICATION
A recent study showed that the ways in which physicians communicate with their patients significantly affected the length of time patients stayed on medication. Patients who were told to take their medication for less than 6 months were much more likely to discontinue medication compared with those who were told to take the medication for 6 months or more. Patients were also more likely to stay on medication if physicians discussed adverse effects of medication. In addition, patients seeing their physician 3 times or more after starting medication significantly decreased the odds of discontinuing medication.17
Patients with depression should have at least 3 follow-up visits during the first 12 weeks of treatment.18 The longer patients stay on medication, the more likely they are to get to remission.
SWITCHING ANTIDEPRESSANTS
The vast majority of depressed patients seen in primary care settings are initially started on an SSRI because of their ease of use; broad efficacy in comorbid anxiety and anxiety disorders; and their significant advantage in terms of side effects when compared to their predecessors, the tricyclic antidepressants (TCAs). There is no evidence that, as a class, SSRIs have any advantage in terms of efficacy (when defined as response) when compared to any of the other antidepressant agents.
The American Psychiatric Association states that all antidepressants approved by the FDA are generally considered equally effective.19 This equivalence of efficacy is based on the fact that rates of response to all approved antidepressants are similar in clinical trials. The FDA standard defines response as a 50% reduction in the HAM-D, no matter what the final HAM-D score. As applied in the STAR*D study, the remission is defined as a HAM-D score of 7 or less.
Clinicians who do not document HAM-D scores must rely on asking patients about their symptoms at follow-up visits. Physicians should not stop adjusting medication regimens?increasing the dosage, augmenting with a second medication, or substituting drugs?until all symptoms have completely, or almost completely, abated. While SSRIs may be the preferred first antidepressant, there is evidence that dual reuptake inhibitors may be more likely to get patients to remission.20,21
Switching from one SSRI to another SSRI There is evidence that a patient who does not respond to one SSRI may very well respond to another. Response rates from 42% to 71% have been reported.22,23 SSRIs all theoretically work by selectively increasing the amount of serotonin in the synapse, but there are significant differences in their molecular structure as well as in their pharmacodynamic and pharmacokinetic properties. It follows that a trial of a second SSRI, when a patient is intolerant or unresponsive to one SSRI, is a reasonable option.
Switching to venlafaxine A growing body of evidence demonstrates that patients treated with venlafaxine are more likely to get to remission, defined as complete or near-complete resolution of all symptoms of depression.20,21 This may be due to venlafaxine's ability to inhibit the reuptake of both serotonin and norepinephrine, so-called dual reuptake inhibition. The older TCAs are also dual reuptake inhibitors, but their poor side-effect profile and potential lethality make them less desirable; they are only given as a last resort for most patients. Until recently, venlafaxine was the only non-TCA dual reuptake inhibitor available. It is commonly believed that venlafaxine acts mainly as an SSRI at doses up to 150 mg; above that level the norepinephrine effects are likely to begin. While most patients on venlafaxine are dosed at 75 mg/d, dosages up to 375 mg/d are commonly used. Duloxetine is dosed at 40 to 60 mg/d for the treatment of depression.
Switching to bupropion Bupropion has been shown to be effective in treating depression in one trial in patients who were not responsive to SSRIs; in this case, fluoxetine (Prozac).24 Despite the lack of a significant amount of supporting data, clinicians have been using bupropion as a favored agent to switch to when an SSRI fails.13 Bupropion has also become the most widely used agent for augmentation in patients who only partially respond to an SSRI. Bupropion has a low risk of sexual side effects, and it is also used as an antidote to treat the sexual side effects of SSRIs. Another significant advantage to the use of bupropion is the reduced risk of weight gain.
Switching to mirtazapine The exact mechanism of action of mirtazapine is unknown, but this unique antidepressant antagonizes both alpha2-adrenergic and serotonin receptors. One multicenter study showed a 47% response rate in patients switched to mirtazapine who had failed to tolerate or respond to SSRI treatment.25 Sedation and weight gain are common side effects of mirtazapine.
Switching to nefazodone Patients who have a poor response to SSRIs may improve when switched to nefazodone.26 Nefazodone is also associated with fewer sexual side effects than SSRIs. Its primary drawback is that it is metabolized through the liver, rather than through the cytochrome P-450 3A4 system. This system is responsible for causing serious hepatic dysfunction when two 3A4 drugs are used in combination. Nefazodone is dosed at 300 to 600 mg/d in divided doses. The drug is relatively sedating and often requires dosage titration.
TAPERING OR WASHOUT VERSUS SUBSTITUTION
Table 1 Switching antidepressants
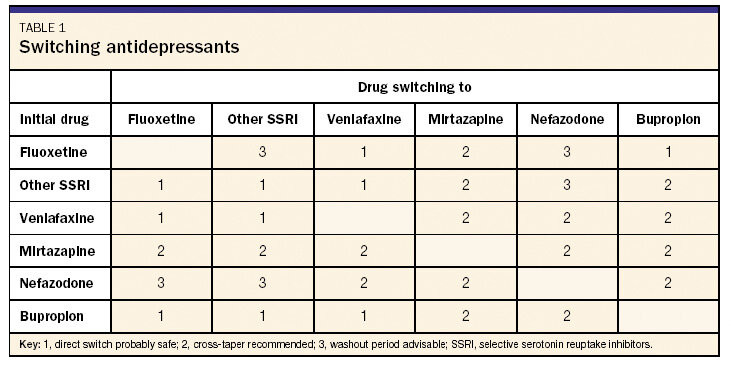
The clinician has several choices in how to switch from one drug to another. There is usually no need to completely stop the first drug and then provide for a washout period before starting the next drug. There are important exceptions, however: It is imperative to have a washout period when changing to or from a monoamine oxidase inhibitor (MAOI). MAOIs used within a few weeks of an SSRI can lead to the serotonin syndrome, a dramatic and potentially fatal condition characterized by confusion, agitation, fever, tachycardia, and tremors. The serotonin syndrome occurs as a result of overstimulation of serotonin receptors. Even though MAOIs are prescribed infrequently in primary care, it is still important to know if a patient has recently used any of these drugs before starting another antidepressant. The other situation where a washout period may be the safest option is when the switch involves an SSRI and nefazodone (see Table 1).
There are 2 options for switching medications: immediate substitution or cross-taper?the gradual introduction of the new drug while tapering the dosage of the first. In most cases, especially when changing from one SSRI to another, one drug can immediately be substituted for another. The risk of serotonin syndrome is relatively small, since the half-lives of SSRIs are relatively short. The only exception is fluoxetine, which has a half-life of 1 to 4 days and an active metabolite with a half-life of 7 to 15 days. Theoretically, when switching from fluoxetine to another SSRI, especially in patients who have been on higher dosages of fluoxetine for any significant length of time, it may be safest to consider stopping the fluoxetine for a few days before beginning the new drug.
A direct switch between SSRIs and venlafaxine is acceptable because venlafaxine inhibits serotonin uptake. Mirtazapine, however, has a different mechanism of action from the SSRIs, so a cross-taper is usually recommended when switching between mirtazapine and an SSRI. There are case reports of serotonin syndrome in patients taking an SSRI with nefazodone.27 The safest approach to changing to or from nefazodone to or from an SSRI would include tapering the first medication (usually by 50% every 5 days or by 1 dosage level each week) and then allowing for a few days' washout before starting the new medication.
Table 2 Discontinuation syndrome
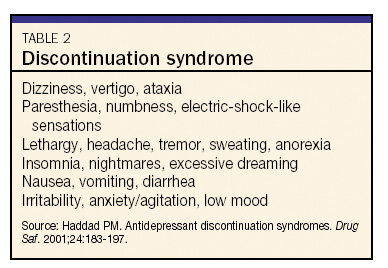
One possible complication of changing from one antidepressant to another is discontinuation syndrome.28 The only SSRI not associated with a discontinuation syndrome is fluoxetine, which, because of its long half-life, tapers itself over a long period of time. Many different symptoms have been reported in patients who abruptly stop taking an antidepressant (see Table 2). This syndrome is most common in switching from an SSRI or venlafaxine to bupropion, since bupropion appears to inhibit the reuptake of norepinephrine and dopamine but is only a weak inhibitor of serotonin reuptake. To avoid discontinuation syndrome, taper an SSRI or venlafaxine while starting a patient on bupropion. There seems to be less risk of discontinuation syndrome when changing a patient's regimen from an SSRI to mirtazapine.
REFERENCES
*Available in generic formulationin the US; the brand Serzone is no longer marketed in the US or Canada.
Jan 1, 2007
By KEVIN SCOTT FERENTZ, MD
Patients with depression who previously did not achieve remission with antidepressant drug therapy can be helped with an additional medication or a different antidepressant according to the STAR*D study. By learning how to switch from one drug to another, you can help more patients attain complete relief from their depression.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study released in 2006, the nation's largest depression study, funded by the National Institutes of Health's National Institute of Mental Health (NIMH), shows that people whose depression is resistant to initial treatment can achieve remission and symptom relief when treated with a secondary strategy that either augments or switches medications. STAR*D is the first study to examine the effectiveness of different treatment strategies for those who did not become symptom free after initial medication.1
An overall assessment of the study suggests that a patient with persistent depression can get well after trying several treatment strategies, but his or her odds of beating the depression diminish as additional treatment strategies are needed.1
The goal of the treatment of depression is remission, meaning the complete or near-complete resolution of all symptoms.2 Current guidelines suggest the acute phase of treatment?when medication is started?generally lasts for up to 12 weeks. In the acute phase, the treatment goal is to get the patient into remission, the point at which symptoms are completely, or nearly completely, gone. Once patients are in remission, they enter the maintenance phase of treatment, which should last for an additional 4 to 9 months.3 The problem is that many patients do not remain on medication long enough to reach remission, let alone stay on medication for the additional recommended months. Up to 33% of patients stop medication within the first month, and up to 44% discontinue medication within 3 months of initiating therapy.4-6
In the STAR*D trial, 3600 adults with nonpsychotic major depressive disorder received 1 to 4 successive acute treatment steps. Those who achieved symptom remission could enter a 12-month naturalistic follow-up phase. Remission was defined as a score of 5 or less on the Quick Inventory of Depressive Symptomatology-Self-Report or a score of 7 or less on the Hamilton Rating Scale for Depression (HAM-D).1 Remission rates in the STAR*D trial were 37%, 31%, 14%, and 13% for the first, second, third, and fourth acute treatment steps, respectively. In step 1, subjects received citalopram (Celexa) as their first treatment step. Step 2 provided 7 possible treatments involving 4 switch treatments?citalopram was stopped and new treatment initiated with sustained-release bupropion (Wellbutrin), cognitive therapy, sertraline (Zoloft), or extended-release venlafaxine (Effexor) and 3 augmentation options (citalopram plus bupropion, buspirone [BuSpar], or cognitive therapy). Step 3 included 2 medication switch strategies (mirtazapine [Remeron] or nortriptyline [Pamelor]) or 2 medication augmentation strategies (lithium or T3). Level 3 entailed randomization to either tranylcypromine (Parnate) or extended-release venlafaxine. The investigators conclude that lower acute remission rates and higher relapse rates can be expected when more treatment steps are needed.1
Two of the many reasons patients discontinue antidepressant medication are intolerance to the medication (adverse events) and lack of response (either partial or complete). When a patient is having intolerable side effects, or when a patient is not responding to an antidepressant, clinicians often choose to switch patients from one antidepressant to another.
SIDE EFFECTS OF ANTIDEPRESSANTS
Side effects from antidepressant medication include those that occur during the acute phase of treatment, as well as those that become problematic during long-term treatment, such as weight gain and sexual dysfunction. Consequently, comparing side-effect profiles of different antidepressants often requires comparing combinations of different clinical trials, all of which use different methods of reporting side effects. As a result, it is difficult to get a true picture of the adverse effects one can expect from any single antidepressant in an individual patient. Many beliefs about the side-effect profiles of antidepressants are based on the marketing data of pharmaceutical companies and our own personal experiences with small numbers of patients.
MANAGING COMMON SIDE EFFECTS
Generally speaking, the adverse effects that are most likely to necessitate a change in medication occur early in treatment; the exceptions are the late-onset side effects of weight gain and sexual dysfunction. The most common early adverse effects of selective serotonin reuptake inhibitors (SSRIs) are nausea and headache. But these effects are usually relatively mild, and usually resolve within 2 to 4 weeks.7 Taking medications with meals or at bedtime often helps minimize these side effects. The SSRIs, as a class, are generally sedating, with paroxetine (Paxil), the most anticholinergic, often considered the most sedating of the group. Mirtazapine and trazodone (Desyrel) are also highly sedating, while nefazodone is somewhat less so.
If patients find that their medication has a sedative effect, they should be told to take it at bedtime. Bupropion is the only antidepressant that can cause insomnia in more than a few patients. Even though this effect is seen much less often with the longer-acting formulations, because of its potential activating effect, bupropion should generally be taken in the morning. The side-effect profile of venlafaxine, a serotonin and norepinephrine reuptake inhibitor, is much like that of the SSRIs. At higher doses, however, it can cause hypertension. This is believed to be due to the enhanced reuptake inhibition of norepinephrine at doses of 150 mg and higher. Approximately 3% of patients treated with 75 to 375 mg/d of sustained-release venlafaxine experience nontransient BP.8 Duloxetine (Cymbalta), a recently released dual reuptake inhibitor, appears less likely to cause hypertension.
Up to 60% of patients taking an SSRI will experience some degree of sexual dysfunction.7 This may manifest as delayed orgasm, inability to have an orgasm, and/or decreased libido. Manufacturers may claim different rates of sexual dysfunction for their various products, but this adverse effect appears to be a class phenomenon. A large multicenter trial based in primary care practices revealed that all SSRIs, venlafaxine, and mirtazapine caused similar rates of sexual dysfunction, while bupropion and nefazodone were least likely to cause sexual dysfunction.9 A recent review details the various pharmacologic strategies for dealing with this common problem.10 Weight gain?averaging 3 to 4 kg?is also a common late side effect of antidepressants. Nutritional counseling and exercise are usually the only measures necessary to deal with this problem. Mirtazapine is associated with larger amounts of weight gain.
PARTIAL AND NO RESPONSE
In most cases, patients placed on a reasonable dosage of an antidepressant should show some response after 4 weeks. If patients have shown no response after 3 to 4 weeks on an antidepressant, there is less than a 20% chance they will show a clinical response after 6 to 8 weeks.11 Overall, approximately 29% to 46% of depressed patients will show only partial or no response to antidepressant treatment.12 A more specific estimate is that 12% to 15% of patients will partially respond and 19% to 34% will not respond at all to a given antidepressant medication.12
Options available to patients who do not achieve remission on a single antidepressant include dosage increase, augmentation with a second drug, and switching to a new medication. In a survey of 432 attendees (mostly psychiatrists) at a psychopharmacology course, clinicians were asked what they would do when faced with a patient who either partially responded or did not respond to 8 weeks of treatment with an SSRI.13 The most common first-choice treatment strategy for partial responders was to raise the dosage of the SSRI. For nonresponders, the most popular choice was to switch to a non-SSRI antidepressant. There are data that indicate switching from one SSRI to another may be beneficial if a patient is either intolerant to or not responding to one particular SSRI.14,15 There is also evidence that suggests that it can be useful to switch to a non-SSRI when faced with a nonresponder.
The issue of augmentation versus switching is beyond the scope of this article but was recently addressed by a large expert panel.16 The panel concluded that "simultaneous targeting of both the noradrenergic and serotonergic systems is one of the most effective augmentation strategies." The panel also recommended switching to an agent of a different class in patients who fail to respond to first-line therapy.
HOW TO KEEP PATIENTS ON MEDICATION
A recent study showed that the ways in which physicians communicate with their patients significantly affected the length of time patients stayed on medication. Patients who were told to take their medication for less than 6 months were much more likely to discontinue medication compared with those who were told to take the medication for 6 months or more. Patients were also more likely to stay on medication if physicians discussed adverse effects of medication. In addition, patients seeing their physician 3 times or more after starting medication significantly decreased the odds of discontinuing medication.17
Patients with depression should have at least 3 follow-up visits during the first 12 weeks of treatment.18 The longer patients stay on medication, the more likely they are to get to remission.
SWITCHING ANTIDEPRESSANTS
The vast majority of depressed patients seen in primary care settings are initially started on an SSRI because of their ease of use; broad efficacy in comorbid anxiety and anxiety disorders; and their significant advantage in terms of side effects when compared to their predecessors, the tricyclic antidepressants (TCAs). There is no evidence that, as a class, SSRIs have any advantage in terms of efficacy (when defined as response) when compared to any of the other antidepressant agents.
The American Psychiatric Association states that all antidepressants approved by the FDA are generally considered equally effective.19 This equivalence of efficacy is based on the fact that rates of response to all approved antidepressants are similar in clinical trials. The FDA standard defines response as a 50% reduction in the HAM-D, no matter what the final HAM-D score. As applied in the STAR*D study, the remission is defined as a HAM-D score of 7 or less.
Clinicians who do not document HAM-D scores must rely on asking patients about their symptoms at follow-up visits. Physicians should not stop adjusting medication regimens?increasing the dosage, augmenting with a second medication, or substituting drugs?until all symptoms have completely, or almost completely, abated. While SSRIs may be the preferred first antidepressant, there is evidence that dual reuptake inhibitors may be more likely to get patients to remission.20,21
Switching from one SSRI to another SSRI There is evidence that a patient who does not respond to one SSRI may very well respond to another. Response rates from 42% to 71% have been reported.22,23 SSRIs all theoretically work by selectively increasing the amount of serotonin in the synapse, but there are significant differences in their molecular structure as well as in their pharmacodynamic and pharmacokinetic properties. It follows that a trial of a second SSRI, when a patient is intolerant or unresponsive to one SSRI, is a reasonable option.
Switching to venlafaxine A growing body of evidence demonstrates that patients treated with venlafaxine are more likely to get to remission, defined as complete or near-complete resolution of all symptoms of depression.20,21 This may be due to venlafaxine's ability to inhibit the reuptake of both serotonin and norepinephrine, so-called dual reuptake inhibition. The older TCAs are also dual reuptake inhibitors, but their poor side-effect profile and potential lethality make them less desirable; they are only given as a last resort for most patients. Until recently, venlafaxine was the only non-TCA dual reuptake inhibitor available. It is commonly believed that venlafaxine acts mainly as an SSRI at doses up to 150 mg; above that level the norepinephrine effects are likely to begin. While most patients on venlafaxine are dosed at 75 mg/d, dosages up to 375 mg/d are commonly used. Duloxetine is dosed at 40 to 60 mg/d for the treatment of depression.
Switching to bupropion Bupropion has been shown to be effective in treating depression in one trial in patients who were not responsive to SSRIs; in this case, fluoxetine (Prozac).24 Despite the lack of a significant amount of supporting data, clinicians have been using bupropion as a favored agent to switch to when an SSRI fails.13 Bupropion has also become the most widely used agent for augmentation in patients who only partially respond to an SSRI. Bupropion has a low risk of sexual side effects, and it is also used as an antidote to treat the sexual side effects of SSRIs. Another significant advantage to the use of bupropion is the reduced risk of weight gain.
Switching to mirtazapine The exact mechanism of action of mirtazapine is unknown, but this unique antidepressant antagonizes both alpha2-adrenergic and serotonin receptors. One multicenter study showed a 47% response rate in patients switched to mirtazapine who had failed to tolerate or respond to SSRI treatment.25 Sedation and weight gain are common side effects of mirtazapine.
Switching to nefazodone Patients who have a poor response to SSRIs may improve when switched to nefazodone.26 Nefazodone is also associated with fewer sexual side effects than SSRIs. Its primary drawback is that it is metabolized through the liver, rather than through the cytochrome P-450 3A4 system. This system is responsible for causing serious hepatic dysfunction when two 3A4 drugs are used in combination. Nefazodone is dosed at 300 to 600 mg/d in divided doses. The drug is relatively sedating and often requires dosage titration.
TAPERING OR WASHOUT VERSUS SUBSTITUTION
Table 1 Switching antidepressants

The clinician has several choices in how to switch from one drug to another. There is usually no need to completely stop the first drug and then provide for a washout period before starting the next drug. There are important exceptions, however: It is imperative to have a washout period when changing to or from a monoamine oxidase inhibitor (MAOI). MAOIs used within a few weeks of an SSRI can lead to the serotonin syndrome, a dramatic and potentially fatal condition characterized by confusion, agitation, fever, tachycardia, and tremors. The serotonin syndrome occurs as a result of overstimulation of serotonin receptors. Even though MAOIs are prescribed infrequently in primary care, it is still important to know if a patient has recently used any of these drugs before starting another antidepressant. The other situation where a washout period may be the safest option is when the switch involves an SSRI and nefazodone (see Table 1).
There are 2 options for switching medications: immediate substitution or cross-taper?the gradual introduction of the new drug while tapering the dosage of the first. In most cases, especially when changing from one SSRI to another, one drug can immediately be substituted for another. The risk of serotonin syndrome is relatively small, since the half-lives of SSRIs are relatively short. The only exception is fluoxetine, which has a half-life of 1 to 4 days and an active metabolite with a half-life of 7 to 15 days. Theoretically, when switching from fluoxetine to another SSRI, especially in patients who have been on higher dosages of fluoxetine for any significant length of time, it may be safest to consider stopping the fluoxetine for a few days before beginning the new drug.
A direct switch between SSRIs and venlafaxine is acceptable because venlafaxine inhibits serotonin uptake. Mirtazapine, however, has a different mechanism of action from the SSRIs, so a cross-taper is usually recommended when switching between mirtazapine and an SSRI. There are case reports of serotonin syndrome in patients taking an SSRI with nefazodone.27 The safest approach to changing to or from nefazodone to or from an SSRI would include tapering the first medication (usually by 50% every 5 days or by 1 dosage level each week) and then allowing for a few days' washout before starting the new medication.
Table 2 Discontinuation syndrome

One possible complication of changing from one antidepressant to another is discontinuation syndrome.28 The only SSRI not associated with a discontinuation syndrome is fluoxetine, which, because of its long half-life, tapers itself over a long period of time. Many different symptoms have been reported in patients who abruptly stop taking an antidepressant (see Table 2). This syndrome is most common in switching from an SSRI or venlafaxine to bupropion, since bupropion appears to inhibit the reuptake of norepinephrine and dopamine but is only a weak inhibitor of serotonin reuptake. To avoid discontinuation syndrome, taper an SSRI or venlafaxine while starting a patient on bupropion. There seems to be less risk of discontinuation syndrome when changing a patient's regimen from an SSRI to mirtazapine.
REFERENCES
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J. Psychiatry. 2006;163:1905-1917.
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder [revision]. Am J Psychiatry. 2000; 157(4 suppl):1-45.
- Depression Guideline Panel. Depression in Primary Care, Vol. 2: Treatment of Major Depression. Rockville, Md: US Dept of Health and Human Services, Public Health Service, and Agency for Health Care Policy and Research; 1993. Clinical Practice Guideline No. 5.
- Simon GE, VonKorff M, Wagner EH, et al. Patterns of antidepressant use in community practice. Gen Hosp Psychiatry. 1993;15:399-408.
- Venturini F, Sung J, Nichol M, et al. Utilization patterns of antidepressant medications in a patient population served by a primary care medical group. J Manag Care Pharm. 1999;5:243-249.
- Lin EH, VonKorff M, Lin E, et al. The role of the primary care physician in patient's adherence to antidepressant therapy. Med Care. 1995;33:67-74.
- Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69-84.
- Effexor SR [package insert]. Collegeville, Pa: Wyeth; 2004.
- Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63:357-366.
- Keltner NL, McAfee KM, Taylor CL. Mechanisms and treatments of SSRI-induced sexual dysfunction. Perspectives in Psychiatr Care. 2002;38:111-116.
- Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry. 1995;152:1500-1503.
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179-200.
- Fredman SF, Fava M, Kienke AS, et al. Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: a survey of current "next-step" practices. J Clin Psychiatry. 2000;61:403-408.
- Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry. 1997;58:16-21.
- Brown A, Harrison W. Are patients who are intolerant to one serotonin selective reuptake inhibitor intolerant to another? J Clin Psychiatry. 1995;56:30-34.
- Hirschfeld RM, Montgomery SA, Aguglia E, et al. Partial response and nonresponse to antidepressant therapy: current approaches and treatment options. J Clin Psychiatry. 2002;63:826-837.
- Bull SA, Hu H, Hunkeler EM, et al. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA. 2002;288:1403-1409.
- National Committee for Quality Assurance. HEDIS 2000: Technical Specifications. Vol 2. Washington, DC: National Committee for Quality Assurance; 1999:105-110.
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(suppl 4):1-45.
- Thase ME. Effectiveness of antidepressants: comparative remission rates. J Clin Psychiatry. 2003;64(suppl 2):3-7.
- Nemeroff CB, Entsuah AR, Willard L, et al. Venlafaxine and SSRIs: pooled remission analysis. Eur Neuropsychopharmacol. 2003;13(suppl 4):S254. Abstract P.1.189. doi:10.1016/S0924-977X(03)91899-2.
- Zarate CA Jr, Kando JC, Tohen M, et al. Does intolerance or lack of response with fluoxetine predict the same will happen with sertraline? J Clin Psychiatry. 1996;57:68-71.
- Joffe RT, Levitt AJ, Sokolov ST, et al. Response to an open trial of a second SSRI in major depression. J Clin Psychiatry. 1996;57:114-115.
- Fava M, Papakostas GI, Petersen T, et al. Switching to bupropion in fluoxetine-resistant major depressive disorder. Ann Clin Psychiatry. 2003;15:17-22.
- Fava M, Dunner DL, Griest JH, et al. An open-label study with mirtazapine in depressed patients who are SSRI treatment failures. Presented at: New Research Program and Abstracts of the 152nd Annual Meeting of the American Psychiatric Association; May 19, 1999; Washington, DC. Abstract NR431:186.
- Thase ME, Zajecka J, Kornstein SG, et al. Nefazodone treatment of patients with poor response to SSRI's. Presented at: 37th annual meeting of the American College of Neuropsychopharmacology (ACNP); December 14-18, 1998; Los Croabas, Puerto Rico.
- John L, Perreault MM, Tao T, et al. Serotonin syndrome associated with nefazodone and paroxetine. Ann Emerg Med. 1997;29:287-289.
- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24:183-197.
- Bupropion (Wellbutrin)
- Buspirone (BuSpar)
- Citalopram (Celexa)
- Duloxetine (Cymbalta)
- Fluoxetine (Prozac)
- Mirtazapine (Remeron)
- Nefazodone*
- Nortriptyline (Pamelor)
- Paroxetine (Paxil)
- Sertraline (Zoloft)
- Tranylcypromine (Parnate)
- Trazodone (Desyrel)
- Venlafaxine (Effexor)
*Available in generic formulationin the US; the brand Serzone is no longer marketed in the US or Canada.
