David Baxter PhD
Late Founder
The Effects of Antidepressants on Sleep: The Good and the Bad
By Andrew Winokur, MD, PhD, and Nicholas DeMartinis, MD, Psychiatric Times
June 13, 2012
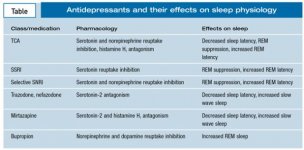
Many psychiatric disorders are accompanied by disturbance of sleep. In addition to resolving sleep-related symptoms through their primary therapeutic effects, many psychiatric medications have secondary effects on sleep that can contribute to their overall therapeutic benefit or sometimes counter them through adverse effects. The antidepressants are a prototypical example of the potentially complex interactions between psychiatric medications and sleep. It is thought that all approved antidepressants work through modulation of monoamine neurotransmitters, including norepinephrine(Drug information on norepinephrine), dopamine(Drug information on dopamine), and serotonin, all of which have been shown to exert prominent effects in regulating sleep-wakefulness and sleep architecture (Table).1 In particular, norepinephrine and serotonin play prominent roles in suppressing REM sleep, while acetylcholine (ACh) plays a key role in the initiation of REM sleep.2 A number of antidepressants affect other neurotransmitter receptors, such as muscarinic ACh, α[SUB]1[/SUB]-adrenergic, and histamine H[SUB]1[/SUB]receptors, that are implicated in sleep regulation.3
TCAs
Pharmacological differences among TCAs translate into clinically differentiating features. TCAs such as amitriptyline(Drug information on amitriptyline) and doxepin(Drug information on doxepin) are used in depressed patients who have prominent insomnia. In recent years, clinicians have scaled down the dosages of these antidepressants to well below established ranges to treat insomnia symptoms. Pharmacologically, the TCAs noted for their sleep-enhancing effects are more potent in blocking the serotonin transporter than the norepinephrine transporter, in addition to exerting prominent blockade of histamine H[SUB]1 [/SUB]receptors.3 Doxepin has been studied at very low dosages in patients with primary insomnia. The studies demonstrate the efficacy of low-dosage doxepin therapy (3 and 6 mg qhs) for alleviating symptoms of insomnia.4 Doxepin is now marketed in the US as Silenor, in 3- and 6-mg dose strengths for primary insomnia.
Some TCAs are more activating and have been used to treat a subset of depressed patients who experience hypersomnolence and daytime lethargy. Examples of TCAs in this category include desipramine and protriptyline, agents that are characterized by the selective blockade of norepinephrine presynaptic uptake sites.3 In polysomnographic studies of patients with depression, administration of the activating TCA desipramine has been reported to produce an increase in sleep onset latency, a decrease in sleep efficiency, and a heightened number of awakenings.5 These findings underscore the important lesson that treatment with an antidepressant does not automatically result in improvement in sleep in depressed patients.
The majority of TCAs markedly suppress REM sleep, except for trimipramine, trazodone, nefazodone(Drug information on nefazodone), bupropion, and mirtazapine(Drug information on mirtazapine).1,6 A clinical consequence of REM suppression can be a change in frequency and intensity of dreaming, as well as a pronounced exacerbation of intense, disturbing dreams related to ?REM rebound? on discontinuation. Pulmonary specialists sometimes advocate use of an activating TCA such as protriptyline because it may help suppress REM sleep?when sleep apnea episodes may be accentuated?and also provide benefit for the daytime somnolence that many patients with sleep apnea experience.7
MAOIs
These agents increase concentrations of norepinephrine, dopamine, and serotonin by decreasing their metabolism.8While the MAOIs have demonstrated efficacy in the treatment of depression in general, they especially have been associated with efficacy in the treatment of atypical depression characterized by hypersomnolence as well as by apathy and low energy.9
Tranylcypromine is structurally related to amphetamine and tends to be activating and associated with insomnia. In polysomnographic studies, tranylcypromine has been demonstrated to prolong sleep onset latency and increase awakenings and arousals during sleep.10 Phenelzine(Drug information on phenelzine) is more typically sedating and is less frequently associated with complaints of insomnia. Notably, both have been demonstrated to produce REM suppression, and discontinuation of treatment can be associated with significant REM rebound.6 Selegiline(Drug information on selegiline) is now available as a skin patch, which may be less likely to cause insomnia.
SSRIs
The SSRIs are characterized by selective inhibition of the presynaptic serotonin transporter, leading to enhanced activity of serotonin at postsynaptic receptors.3 A large number of serotonin receptor subtypes that regulate sleep and wakefulness as well as transitions between specific sleep stages, such as the termination of REM sleep, have been identified.
Because of the complexity of serotonin involvement in sleep-wake regulation, drugs that modulate serotonin activity can produce prominent and sometimes diverse effects on sleep. Some patients who took fluoxetine(Drug information on fluoxetine) reported insomnia as an adverse effect, whereas other patients experienced daytime somnolence.11 This same pattern of diverse subjective reports on sleep and wakefulness has been reported in clinical trials with all of the drugs in this class.
While data have been reported most extensively for fluoxetine and paroxetine(Drug information on paroxetine), class effects of SSRI therapy appear to include increased sleep onset latency and/or an increased number of awakenings and arousals, leading to an overall decrease in sleep efficiency.12,13 Virtually all of the SSRIs examined have been noted to suppress REM sleep.1 Clinically, reports of a change in the frequency, intensity, and content of dreaming can be associated with SSRIs, as well as the occurrence of these symptoms on discontinuation.
Be mindful that treatment of a patient with depression may produce significant improvement in symptoms of depression in general, yet may not address insomnia. In some cases, treatment with an SSRI may produce or exacerbate problems with sleep disturbance. Therefore, a medication that targets insomnia may also be prescribed for patients with depression who are being treated with an SSRI.
Selective SNRIs
By inhibiting both the serotonin and norepinephrine transporters, selective SNRIs engage a broader mechanism of action than do SSRIs.8 While significantly fewer polysomnographic studies have been conducted with the selective SNRIs, the general pattern of effects reported are comparable to those seen with SSRIs, including the potential for some disruption of sleep continuity and prominent suppression of REM sleep.1 A recent clinical application of antidepressants to clinical sleep disorders has stemmed from the recognition that broad-spectrum antidepressants have value in the treatment of fibromyalgia.14 Selective SNRIs have been used in patients with this disorder, and the selective SNRI milnacipran has been approved and is now marketed for fibromyalgia.
Atypical antidepressants
Trazodone is characterized by serotonin-2 receptor antagonism and weak serotonin reuptake inhibition.8 This effect is noteworthy with respect to sleep physiology, since an increase of serotonin neurotransmission at the serotonin-2 receptor can result in disruption of sleep continuity as well as inhibition of slow wave sleep. In contrast, serotonin-2 receptor antagonists have been shown to decrease sleep onset latency and increase slow wave sleep.
Trazodone also blocks histamine H[SUB]1[/SUB] receptors, which can be associated with enhanced sleep and potential for daytime somnolence.15 Trazodone was introduced as an antidepressant with a dosage range of 200 to 600 mg/d in 2 or 3 divided doses. However, trazodone produced prominent daytime somnolence in this dosage range, which made it an unacceptable option for many patients.
Clinicians soon came to recognize that by scaling down the dosage substantially (typically to a range of 50 to 100 mg qhs), trazodone could be used as an adjunctive treatment for improving symptoms of insomnia without causing significant daytime somnolence. Thus, trazodone is now frequently used off-label as add-on therapy to other antidepressant drugs to address residual insomnia.1,16 In some polysomnographic studies, trazodone was seen to increase total sleep time and reduce wakefulness during sleep. In addition, as noted above, trazodone appears to exert minimal effects in suppressing REM sleep.
Nefazodone is structurally similar to trazodone and has a relatively similar pharmacological profile.8 In polysomnographic studies involving depressed patients with prominent insomnia complaints, nefazodone was reported to preserve sleep continuity and to be devoid of REM suppression.12 The clinical use of nefazodone in recent years has been significantly limited by reports of liver toxicity, in some cases involving fatalities. Even though the incidence of this complication is extremely uncommon, its potential severity appears to have significantly reduced nefazodone use in the treatment of depression despite its beneficial sleep profile.
Mirtazapine demonstrates a unique pharmacological profile among the antidepressant drugs, characterized by inhibition of the presynaptic α[SUB]2[/SUB]-adrenergic receptor as well as blockade of serotonin-2 and serotonin-3 receptors and histamine H[SUB]1[/SUB]receptors.8 The combination of serotonin-2 and histamine H[SUB]1[/SUB] receptor blockade provides a strong basis to anticipate a profile of sleep enhancement; however, there is also potential for daytime somnolence. Polysomnographic studies in depressed patients with prominent insomnia symptoms have reported significant shortening of sleep onset latency and an increase in total sleep time across the full spectrum of mirtazapine?s antidepressant dosage range, from 15 to 45 mg/d.17 There is also limited evidence of REM suppression with mirtazapine therapy.
Currently, mirtazapine may be selected as monotherapy for depressed patients who have prominent associated insomnia complaints. In other depressed patients, mirtazapine may be added to another primary antidepressant drug to augment the antidepressant response and to address unresolved insomnia. Mirtazapine may be associated with daytime somnolence, which usually resolves after a few days of treatment in some cases but which may be more persistent for some patients. Another vexing problem for some patients treated with mirtazapine is weight gain, which may be significant.
Bupropion is somewhat unique among antidepressant drugs in exerting negligible effects on any parameter of serotonin neurotransmission. Bupropion is selective in blocking presynaptic reuptake of dopamine and norepinephrine, but its potency in blocking dopamine and norepinephrine reuptake is limited.3 Increased catecholamine neurotransmission following administration of bupropion appears to lead to complaints of insomnia in some cases.6 Notably, bupropion does not suppress REM sleep, as do most antidepressant drugs, but it actually results in an increase in REM sleep time.18
Conclusion
Neurotransmitters implicated in the mechanism of action of all currently marketed antidepressants have been demonstrated to play important roles in the neurobiological regulation of sleep and wakefulness as well as in the regulation of transitions between the various stages of sleep. In addition, changes in sleep patterns are observed in a large percentage of patients with depression, and unresolved problems with insomnia have been reported to represent the most significant risk factor among residual symptoms of depression in patients who have responded to antidepressant drug therapy.
Knowledge of how different antidepressants are likely to affect parameters of sleep?including latency to sleep onset, maintenance of sleep continuity, and alterations in sleep architecture?can provide an important basis for selecting an appropriate antidepressant drug among the roughly 2 dozen marketed options to most suitably meet the needs of depressed patients.
See also:
References
1. DeMartinis NA, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6:17-29.
2. Hobson JA. Sleep mechanisms and pathophysiology: some clinical implications of the reciprocal interaction hypothesis of sleep cycle control. Psychosom Med. 1983;45:123-140.
3. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 suppl 2):1S-9S.
4. Roth T, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep. 2007;30:1555-1561.
5. Kupfer DJ, Perel JM, Pollock BG, et al. Fluvoxamine versus desipramine: comparative polysomnographic effects. Biol Psychiatry. 1991;29:23-40.
6. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28.
7. Brownell LG, West P, Sweatman P, et al. Protriptyline in obstructive sleep apnea: a double-blink trial. N Engl J Med. 1982;307:1037-1042.
8. Stahl SM. Stahl?s Essential Psychopharmacology. 3rd ed. New York: Cambridge University Press; 2008.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry Suppl. 1993;Sep(21):30-34.
10. Kupfer DJ, Bowers MB Jr. REM sleep and central monoamine oxidase inhibition. Psychopharmacologia. 1972;27:183-190.
11. Beasley CM Jr, Sayler ME, Weiss AM, Potvin JH. Fluoxetine: activating and sedating effects at multiple fixed doses. J Clin Psychopharmacol. 1992;12:328-333.
12. Rush AJ, Armitage R, Gillin JC, et al. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol Psychiatry. 1998;44:3-14.
13. Sharpley AL, Williamson DJ, Attenburrow ME, et al. The effects of paroxetine and nefazodone on sleep: a placebo controlled trial. Psychopharmacology (Berl). 1996;126:50-54.
14. O?Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15:659-666.
15. Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14:536-546.
16. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
17. Winokur A, DeMartinis NA 3rd, McNally DP, et al. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64:1224-1229.
18. Nofzinger EA, Reynolds CF 3rd, Thase ME, et al. REM sleep enhancement by bupropion in depressed men. Am J Psychiatry. 1995;152:274-276.
By Andrew Winokur, MD, PhD, and Nicholas DeMartinis, MD, Psychiatric Times
June 13, 2012
Many psychiatric disorders are accompanied by disturbance of sleep. In addition to resolving sleep-related symptoms through their primary therapeutic effects, many psychiatric medications have secondary effects on sleep that can contribute to their overall therapeutic benefit or sometimes counter them through adverse effects. The antidepressants are a prototypical example of the potentially complex interactions between psychiatric medications and sleep. It is thought that all approved antidepressants work through modulation of monoamine neurotransmitters, including norepinephrine(Drug information on norepinephrine), dopamine(Drug information on dopamine), and serotonin, all of which have been shown to exert prominent effects in regulating sleep-wakefulness and sleep architecture (Table).1 In particular, norepinephrine and serotonin play prominent roles in suppressing REM sleep, while acetylcholine (ACh) plays a key role in the initiation of REM sleep.2 A number of antidepressants affect other neurotransmitter receptors, such as muscarinic ACh, α[SUB]1[/SUB]-adrenergic, and histamine H[SUB]1[/SUB]receptors, that are implicated in sleep regulation.3

TCAs
Pharmacological differences among TCAs translate into clinically differentiating features. TCAs such as amitriptyline(Drug information on amitriptyline) and doxepin(Drug information on doxepin) are used in depressed patients who have prominent insomnia. In recent years, clinicians have scaled down the dosages of these antidepressants to well below established ranges to treat insomnia symptoms. Pharmacologically, the TCAs noted for their sleep-enhancing effects are more potent in blocking the serotonin transporter than the norepinephrine transporter, in addition to exerting prominent blockade of histamine H[SUB]1 [/SUB]receptors.3 Doxepin has been studied at very low dosages in patients with primary insomnia. The studies demonstrate the efficacy of low-dosage doxepin therapy (3 and 6 mg qhs) for alleviating symptoms of insomnia.4 Doxepin is now marketed in the US as Silenor, in 3- and 6-mg dose strengths for primary insomnia.
Some TCAs are more activating and have been used to treat a subset of depressed patients who experience hypersomnolence and daytime lethargy. Examples of TCAs in this category include desipramine and protriptyline, agents that are characterized by the selective blockade of norepinephrine presynaptic uptake sites.3 In polysomnographic studies of patients with depression, administration of the activating TCA desipramine has been reported to produce an increase in sleep onset latency, a decrease in sleep efficiency, and a heightened number of awakenings.5 These findings underscore the important lesson that treatment with an antidepressant does not automatically result in improvement in sleep in depressed patients.
The majority of TCAs markedly suppress REM sleep, except for trimipramine, trazodone, nefazodone(Drug information on nefazodone), bupropion, and mirtazapine(Drug information on mirtazapine).1,6 A clinical consequence of REM suppression can be a change in frequency and intensity of dreaming, as well as a pronounced exacerbation of intense, disturbing dreams related to ?REM rebound? on discontinuation. Pulmonary specialists sometimes advocate use of an activating TCA such as protriptyline because it may help suppress REM sleep?when sleep apnea episodes may be accentuated?and also provide benefit for the daytime somnolence that many patients with sleep apnea experience.7
MAOIs
These agents increase concentrations of norepinephrine, dopamine, and serotonin by decreasing their metabolism.8While the MAOIs have demonstrated efficacy in the treatment of depression in general, they especially have been associated with efficacy in the treatment of atypical depression characterized by hypersomnolence as well as by apathy and low energy.9
Tranylcypromine is structurally related to amphetamine and tends to be activating and associated with insomnia. In polysomnographic studies, tranylcypromine has been demonstrated to prolong sleep onset latency and increase awakenings and arousals during sleep.10 Phenelzine(Drug information on phenelzine) is more typically sedating and is less frequently associated with complaints of insomnia. Notably, both have been demonstrated to produce REM suppression, and discontinuation of treatment can be associated with significant REM rebound.6 Selegiline(Drug information on selegiline) is now available as a skin patch, which may be less likely to cause insomnia.
SSRIs
The SSRIs are characterized by selective inhibition of the presynaptic serotonin transporter, leading to enhanced activity of serotonin at postsynaptic receptors.3 A large number of serotonin receptor subtypes that regulate sleep and wakefulness as well as transitions between specific sleep stages, such as the termination of REM sleep, have been identified.
Because of the complexity of serotonin involvement in sleep-wake regulation, drugs that modulate serotonin activity can produce prominent and sometimes diverse effects on sleep. Some patients who took fluoxetine(Drug information on fluoxetine) reported insomnia as an adverse effect, whereas other patients experienced daytime somnolence.11 This same pattern of diverse subjective reports on sleep and wakefulness has been reported in clinical trials with all of the drugs in this class.
While data have been reported most extensively for fluoxetine and paroxetine(Drug information on paroxetine), class effects of SSRI therapy appear to include increased sleep onset latency and/or an increased number of awakenings and arousals, leading to an overall decrease in sleep efficiency.12,13 Virtually all of the SSRIs examined have been noted to suppress REM sleep.1 Clinically, reports of a change in the frequency, intensity, and content of dreaming can be associated with SSRIs, as well as the occurrence of these symptoms on discontinuation.
Be mindful that treatment of a patient with depression may produce significant improvement in symptoms of depression in general, yet may not address insomnia. In some cases, treatment with an SSRI may produce or exacerbate problems with sleep disturbance. Therefore, a medication that targets insomnia may also be prescribed for patients with depression who are being treated with an SSRI.
Selective SNRIs
By inhibiting both the serotonin and norepinephrine transporters, selective SNRIs engage a broader mechanism of action than do SSRIs.8 While significantly fewer polysomnographic studies have been conducted with the selective SNRIs, the general pattern of effects reported are comparable to those seen with SSRIs, including the potential for some disruption of sleep continuity and prominent suppression of REM sleep.1 A recent clinical application of antidepressants to clinical sleep disorders has stemmed from the recognition that broad-spectrum antidepressants have value in the treatment of fibromyalgia.14 Selective SNRIs have been used in patients with this disorder, and the selective SNRI milnacipran has been approved and is now marketed for fibromyalgia.
Atypical antidepressants
Trazodone is characterized by serotonin-2 receptor antagonism and weak serotonin reuptake inhibition.8 This effect is noteworthy with respect to sleep physiology, since an increase of serotonin neurotransmission at the serotonin-2 receptor can result in disruption of sleep continuity as well as inhibition of slow wave sleep. In contrast, serotonin-2 receptor antagonists have been shown to decrease sleep onset latency and increase slow wave sleep.
Trazodone also blocks histamine H[SUB]1[/SUB] receptors, which can be associated with enhanced sleep and potential for daytime somnolence.15 Trazodone was introduced as an antidepressant with a dosage range of 200 to 600 mg/d in 2 or 3 divided doses. However, trazodone produced prominent daytime somnolence in this dosage range, which made it an unacceptable option for many patients.
Clinicians soon came to recognize that by scaling down the dosage substantially (typically to a range of 50 to 100 mg qhs), trazodone could be used as an adjunctive treatment for improving symptoms of insomnia without causing significant daytime somnolence. Thus, trazodone is now frequently used off-label as add-on therapy to other antidepressant drugs to address residual insomnia.1,16 In some polysomnographic studies, trazodone was seen to increase total sleep time and reduce wakefulness during sleep. In addition, as noted above, trazodone appears to exert minimal effects in suppressing REM sleep.
Nefazodone is structurally similar to trazodone and has a relatively similar pharmacological profile.8 In polysomnographic studies involving depressed patients with prominent insomnia complaints, nefazodone was reported to preserve sleep continuity and to be devoid of REM suppression.12 The clinical use of nefazodone in recent years has been significantly limited by reports of liver toxicity, in some cases involving fatalities. Even though the incidence of this complication is extremely uncommon, its potential severity appears to have significantly reduced nefazodone use in the treatment of depression despite its beneficial sleep profile.
Mirtazapine demonstrates a unique pharmacological profile among the antidepressant drugs, characterized by inhibition of the presynaptic α[SUB]2[/SUB]-adrenergic receptor as well as blockade of serotonin-2 and serotonin-3 receptors and histamine H[SUB]1[/SUB]receptors.8 The combination of serotonin-2 and histamine H[SUB]1[/SUB] receptor blockade provides a strong basis to anticipate a profile of sleep enhancement; however, there is also potential for daytime somnolence. Polysomnographic studies in depressed patients with prominent insomnia symptoms have reported significant shortening of sleep onset latency and an increase in total sleep time across the full spectrum of mirtazapine?s antidepressant dosage range, from 15 to 45 mg/d.17 There is also limited evidence of REM suppression with mirtazapine therapy.
Currently, mirtazapine may be selected as monotherapy for depressed patients who have prominent associated insomnia complaints. In other depressed patients, mirtazapine may be added to another primary antidepressant drug to augment the antidepressant response and to address unresolved insomnia. Mirtazapine may be associated with daytime somnolence, which usually resolves after a few days of treatment in some cases but which may be more persistent for some patients. Another vexing problem for some patients treated with mirtazapine is weight gain, which may be significant.
Bupropion is somewhat unique among antidepressant drugs in exerting negligible effects on any parameter of serotonin neurotransmission. Bupropion is selective in blocking presynaptic reuptake of dopamine and norepinephrine, but its potency in blocking dopamine and norepinephrine reuptake is limited.3 Increased catecholamine neurotransmission following administration of bupropion appears to lead to complaints of insomnia in some cases.6 Notably, bupropion does not suppress REM sleep, as do most antidepressant drugs, but it actually results in an increase in REM sleep time.18
Conclusion
Neurotransmitters implicated in the mechanism of action of all currently marketed antidepressants have been demonstrated to play important roles in the neurobiological regulation of sleep and wakefulness as well as in the regulation of transitions between the various stages of sleep. In addition, changes in sleep patterns are observed in a large percentage of patients with depression, and unresolved problems with insomnia have been reported to represent the most significant risk factor among residual symptoms of depression in patients who have responded to antidepressant drug therapy.
Knowledge of how different antidepressants are likely to affect parameters of sleep?including latency to sleep onset, maintenance of sleep continuity, and alterations in sleep architecture?can provide an important basis for selecting an appropriate antidepressant drug among the roughly 2 dozen marketed options to most suitably meet the needs of depressed patients.
See also:
- Introduction: Understanding Common Sleep Disorders in Psychiatric Illness
- The Effects of Antidepressants on Sleep
- ADHD and Sleep Disorders in Children
- The Role of Melatonin in the Circadian Rhythm Sleep-Wake Cycle
- The Correlation Between Sleep-Disordered Breathing and Psychiatry
References
1. DeMartinis NA, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6:17-29.
2. Hobson JA. Sleep mechanisms and pathophysiology: some clinical implications of the reciprocal interaction hypothesis of sleep cycle control. Psychosom Med. 1983;45:123-140.
3. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 suppl 2):1S-9S.
4. Roth T, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep. 2007;30:1555-1561.
5. Kupfer DJ, Perel JM, Pollock BG, et al. Fluvoxamine versus desipramine: comparative polysomnographic effects. Biol Psychiatry. 1991;29:23-40.
6. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28.
7. Brownell LG, West P, Sweatman P, et al. Protriptyline in obstructive sleep apnea: a double-blink trial. N Engl J Med. 1982;307:1037-1042.
8. Stahl SM. Stahl?s Essential Psychopharmacology. 3rd ed. New York: Cambridge University Press; 2008.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry Suppl. 1993;Sep(21):30-34.
10. Kupfer DJ, Bowers MB Jr. REM sleep and central monoamine oxidase inhibition. Psychopharmacologia. 1972;27:183-190.
11. Beasley CM Jr, Sayler ME, Weiss AM, Potvin JH. Fluoxetine: activating and sedating effects at multiple fixed doses. J Clin Psychopharmacol. 1992;12:328-333.
12. Rush AJ, Armitage R, Gillin JC, et al. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol Psychiatry. 1998;44:3-14.
13. Sharpley AL, Williamson DJ, Attenburrow ME, et al. The effects of paroxetine and nefazodone on sleep: a placebo controlled trial. Psychopharmacology (Berl). 1996;126:50-54.
14. O?Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15:659-666.
15. Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14:536-546.
16. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
17. Winokur A, DeMartinis NA 3rd, McNally DP, et al. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64:1224-1229.
18. Nofzinger EA, Reynolds CF 3rd, Thase ME, et al. REM sleep enhancement by bupropion in depressed men. Am J Psychiatry. 1995;152:274-276.
