www.medscape.com
Pharmacology Update: Pharmacologic Management of Alzheimer Disease
Deborah Downey, MN RN CNRN CNS ANP
Published: 06/03/2008
Overview
Dementia is a major public health problem now estimated to affect about 5.1 million older adults in the United States and about 15 million worldwide. It is expected that there will be 13 million patients diagnosed with Alzheimer disease (AD) by the year 2050. The Alzheimer's Association anticipates that about 50% of adults older than 85 years of age will have a memory disorder, the largest percentage of those having AD (Alzheimer's Association, 2007). As the number of adults with memory disorders grows, so does the burden of caring for them, in terms of both the dollars spent on nursing facilities and the physical and psychological toll on family caregivers while the patient is still at home. This article reviews the pathophysiology of AD and describes the drug treatment options that are currently available. It is important to note that these drugs are not a cure but are aimed at slowing functional decline in AD patients.
Pathophysiology
Alzheimer disease is a progressive decline in the ability to perform routine activities of daily living (ADLs). Two primary pathologic processes are involved. In the first pathologic process, amyloid plaques, consisting of a sticky protein material, accumulate between cells in the brain and slow cellular transmission. These plaques are found mostly in the hippocampus, the area of the brain involved in formation of memories. The second pathologic process involves neurofibrillary tangles, intracellular collections of twisted protein filaments that destroy cells from within. Neurofibrillary tangles are present mostly in the cortex and hippocampus. These two pathologic processes pair together to wreak havoc within the nervous system, slowing transmission and impairing formation of new memories. The two processes may also be seen in other dementias, such as progressive supranuclear palsy, but are classically paired in AD (Geldmacher, 2001).
In addition to structural changes, alterations also occur in the neurotransmitters themselves, which facilitate neuron-to-neuron transmission. Acetylcholine (ACh), an important neurotransmitter involved in memory formation, is reduced as a result of cellular loss in the basal forebrain where most of the ACh is produced. There is also decreased production of norepinephrine (NE) and serotonin (5-HT), both of which contribute to the behavioral and cognitive changes associated with AD (Desai & Grossberg, 2005).
Diagnosis
Diagnosis of AD is made largely by history but must include loss of memory and one or more of the following: aphasia, apraxia, agnosia, and disturbance in executive functioning (American Psychiatric Association, 1994, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [DSM-IV]). Aphasia is an acquired disturbance of language; apraxia is the inability to order routine motor tasks, such as hair combing or dressing, into an organized way to complete them. Agnosia is the inability to interpret sensory information despite not having a primary sensory deficit. Executive functioning includes tasks such as judgment, complex reasoning, organization of tasks, and insight. In addition, there must be significant impairment in social or occupational function or a significant change from baseline abilities, and these changes must not occur during delirium or in the context of other medical disorders (American Psychiatric Association).
It is important to rule out reversible causes of dementia before making a definitive diagnosis of AD. Misdiagnosis deprives the patient of treatment for what may be a reversible condition. Practice parameters from the American Academy of Neurology (AAN) for diagnosis include testing for B12 deficiency and hypothyroidism, as these are common problems and represent potentially reversible causes of memory impairment in the elderly. Laboratory testing should include B12, folate, and thyroid stimulating hormone assay and testing for metabolic disturbances, including diabetes and electrolyte abnormalities. Although many clinicians still test for prior syphilis exposure, unless the patient has specific risk factors, the AAN does not recommend this as part of routine testing at this time (Knopman et al., 2001).
Regarding structural neuroimaging, AAN does not recommend either computed tomography (CT) or magnetic resonance imaging (MRI) as part of the initial evaluation; however, most clinicians are still obtaining some form of imaging to rule out a structural cause for memory impairment. Volumetric CT, positron emission tomography (PET), or single photon emission computed tomography (SPECT) also are not recommended for routine use as part of the initial evaluation; however, they may be appropriate in individual circumstances. It is important to note that the definitive diagnosis of AD is made only on postmortem tissue examination, but about 85% of those who meet current diagnostic criteria during their lifetime will meet neuropathologic criteria at autopsy (Knopman et al., 2001).
It is also important for the patient and family to determine whether the patient has another of the progressive dementias because the course can be quite different from AD, and making this determination offers the family the ability to plan for future care needs. Table 1 lists other progressive dementias that should be considered.
Table 1. Dementias
Risk factors for AD include age, family history of dementia, lower socioeconomic status, lower educational level, depression or other psychiatric illness, previous head injury, and alcohol abuse. Chronic medical problems such as diabetes, hypertension, ischemic coronary disease, and cerebral disease may worsen an existing memory impairment as well.
Treatment
In treating AD, it is important to recognize that it is a progressive disease. Patients will continue to decline functionally, whether they are treated or untreated. Stopping the functional decline is not a treatment goal because it is now known that neuronal loss has most likely been ongoing for some years before actual recognition of a memory disorder. Symptomatic treatment is aimed at improvement in function. A delay in decline does not necessarily mean neuronal loss is being stopped; treatment is aimed at preserving and facilitating remaining synaptic function, allowing a higher functional level for a longer time. When therapy is discontinued, it can be expected that decline will occur at the same rate as untreated dementia, as the rate of neuronal death is not affected (Geldmacher, 2001). Slowing decline can result in benefit for the patient and caregiver even if it does not manifest as clinical improvement.
Despite the many advances in pharmacologic therapy for neurological disease, 6 years after the Decade of the Brain, there are still only five drugs approved for dementia, although many more are in the research pipeline. The current drugs fall into two classes. The drugs in the acetylcholinesterase class are tacrine (Cognex), donepezil (Aricept), rivastigmine (Exelon), and galantamine (Razadyne). Tacrine, the first drug in this class, caused alterations in liver transaminases and liver failure and, although still available, is not a first-line therapy because there are now other safer choices (Tacrine will not be discussed in this article). Memantine (Namenda), an N-methyl-D-aspartate (NMDA) receptor antagonist, is in a separate class.
An important treatment paradigm for AD is the cholinergic benefit. If loss of ACh is the primary neurotransmitter defect, then increasing production, inhibiting destruction, or activating receptors all should translate into effective therapy (i.e., slow functional decline). ACh is broken down in the synaptic junction by acetylcholinesterase. By inhibiting this breakdown, drugs in the acetylcholinesterase group (donepezil, galantamine, and rivastigmine) prevent destruction of ACh.
In clinical trials for the acetylcholinesterase inhibitors, the cognitive portion of the Alzheimer Disease Assessment Scale (ADAS-Cog) was used to assess cognitive function. With some variability among the trials, the acetylcholinesterase inhibitors improved patients' ADAS-Cog score as compared to placebo. Secondary trial measures included evaluation of patients' abilities to maintain ADLs. Although most clinicians feel it is probably true that patients maintain ADL ability longer, definitive trial evidence of this benefit has yet to emerge. In addition, improvement in behavioral function has been reported, but it is more likely that the slowing of cognitive dysfunction delays the emergence of behavioral disturbance (Cummings, 2004).
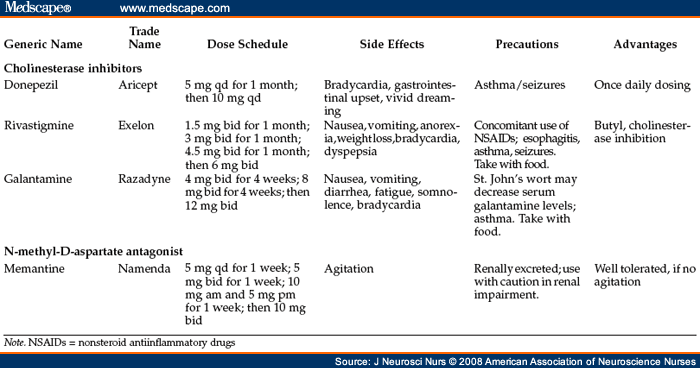
Adverse effects of these drugs are related to their cholinergic properties. Nausea, gastrointestinal (GI) upset, diarrhea, weight loss, bradycardia, syncope, and insomnia can occur. Some patients complain of vivid dreams or nightmares; these symptoms improve with a decrease in dose. Although the drugs have similar profiles, if a patient has difficulty with side effects from one, clinicians sometimes find that another drug in the same class will be better tolerated; however, clinical data to support this observation are lacking. Anticholinergic drugs may inhibit the effect of all the drugs in this class ( Table 2 ).
Table 2. Treatment Options
Donepezil (Aricept) is usually started at 5 mg daily for 1 month and then increased to 10 mg daily. The once-daily dosing is advantageous for patients who live alone or who have difficulty managing medications. Although the package insert advises titration after 1 month, most patients will tolerate a 2-week titration schedule. This drug is metabolized within a minor substrate of the CYP2D6 and 3A4 metabolic system; competing drugs make the drug less available for its therapeutic effect. St. John's Wort may also decrease the drug effect of donepezil. As with all the drugs in the acetylcholinesterase class, GI side effects are the most common; however, slowing the taper up to 10 mg usually improves any adverse GI effects. Donepezil use also appears to cause a higher incidence of vivid dreaming than other drugs in this class, but switching to a morning dose improves sleep quality. Donepezil is now available in an orally disintegrating form as well.
Galantamine (Razadyne) is started at 4 mg bid and is increased to 8 mg and then 12 mg bid at 4-week intervals. Galantamine has recently been approved for a once-daily sustained-release form, and this is now available. This drug also is metabolized within a minor substrate of the CYP2D6 and 3A4 systems, and St. John's Wort may decrease blood levels of donepezil. The name of this drug has recently been changed to Razadyne because its former name, Reminyl, was too similar to that of another drug and was considered to be potentially confusing.
Rivastigmine (Exelon) dosing starts at 1.5 mg bid and is increased to 3 mg bid, then 4.5 mg bid, and then 6 mg bid at 4-week intervals. Rivastigmine has a nonhepatic metabolism, unlike donepezil and galantamine, which are metabolized through the CYP2D6 and CYP3A4 pathways. However, it has been associated with esophageal irritation and should be used with caution in patients with peptic ulcer disease or those using nonsteroidal antiinflammatory drugs. As is the case with the other drugs in this class, cholinergic drugs may potentiate the drug effect, while anticholinergic drugs may inhibit effectiveness. Both rivastigmine and galantamine are available in liquid forms for those patients with swallowing difficulties; no intravenous formulations are available.
One caveat with all the anticholinesterase drugs is that if they are stopped for any reason, they must be restarted at the lowest dose and titrated up again as in the original taper schedules. There have been case reports of esophageal rupture when rivastigmine was stopped and then restarted at the highest tolerated dose.
Memantine (Namenda) is a recently approved NMDA antagonist that is thought to interfere with glutaminergic overstimulation (excitotoxicity). By interfering with excitotoxicity, memantine affects the NMDA receptors implicated in memory processing and the pathology of AD (Reisberg et al., 2003). Clinical trials looked at memantine alone, memantine with placebo, and memantine with donepezil, a cholinesterase inhibitor. The regimens of memantine alone and memantine with donepezil were found to be effective across the domains of cognition, function, behavior, and global status, and the effect was statistically significant (Reisberg et al.). Memantine is currently approved by the U.S. Food and Drug Administration for use in patients with moderate to severe AD. The dosing schedule is 5 mg daily for 1 week, 5 mg bid for 1 week, 10 mg in the morning and 5 mg in the evening for 1 week, and then 10 mg bid. Adverse effects include dizziness, headache, GI upset, and hypertension. There have been case reports of worsening behavioral status and agitation, which have cleared when stopping the drug. Caution also needs to be used when memantine is taken with other NMDA antagonists such as amantadine or dextromethorphan. Memantine undergoes nonhepatic metabolism and is excreted by the kidney, so caution should be taken by patients with renal insufficiency or when other renally excreted drugs are used. Dosages may need to be adjusted or lowered if creatinine rises; sometimes the drug will need to be stopped altogether. Memantine can be taken without regard to meals.
Other Treatment Options
Antioxidant Therapy
Antioxidant therapy has also been studied for AD. In 2001, the AAN Practice Parameter: Management of Dementia (an evidence-based review) suggested adding 1,000 units of vitamin E twice daily as a practice guideline, not a standard (Doody et al., 2001). Despite great hopes, more recent studies have shown that vitamin E has little benefit in influencing the rate of progression from mild cognitive impairment to AD as compared to placebo (Petersen et al., 2005). Less clear is the issue of whether vitamin E slows progression of already diagnosed dementia. Although many clinicians still use vitamin E as additive therapy for patients with AD, its effectiveness remains unclear. A recent study by the Alzheimer's Disease Cooperative Study Group showed that vitamin E changed the likelihood of progressing from mild cognitive impairment to AD at the end of 3 years but that patients on donepezil had a lower rate of progression to AD for the first 12 months. After that, the rate of progression was similar to that of vitamin E. Although 12 months does not seem like a significant amount of time, the impact of that time span on caregivers can be significant from both a caregiving and a financial standpoint by delaying placement in a care facility (Petersen et al.).
Hormone Replacement Therapy
Previous studies and epidemiological reports have suggested that hormone replacement therapy (HRT) might be effective in reducing the onset of cognitive decline. However, the recently published results of the Women's Health Initiative clearly showed an increased risk of dementia among postmenopausal women who had not had any cognitive deficit at enrollment and who were assigned to the treatment group and thus received estrogen (Shumaker et al., 2003). As a result, taking HRT for the prevention of dementia is not currently recommended.
Antiinflammatory Agents
Pathology reviews of brains of patients with AD have shown microscopic inflammation. This finding has led to numerous studies of antiinflammatory agents for AD, including diclofenac, rofecoxib, naproxen, and, most recently, ibuprofen. There is insufficient evidence at this time to support using these drugs as protective agents for AD (Cummings, 2004).
Herbal Supplements
Herbal supplements are often used by patients in conjunction with other prescribed therapies. A frequent choice is ginkgo biloba, whose pharmacologic effect is primarily as a flavoglycoside, a potent free-radical scavenger. It may also function as an antioxidant. A formal review of more than 50 articles in which ginkgo biloba was used for supporting cognitive function showed that on a quantitative analysis of the literature, there was a small but significant effect after 3 to 6 months' treatment with 120?240 mg of ginkgo, but the data were inconsistent (Oken, Storzbach, & Kaye, 1998). There is currently an ongoing primary prevention trial using ginkgo that is studying the rate of development of AD. This may be limited, however, by the possibility of bleeding, as in vitro studies show evidence that ginkgo inhibits platelet aggregation (Basch & Ulbicht, 2005).
Summary
Although the diagnosis of AD can be devastating, treatment options exist that can slow the disease's progression and allow patients to continue performing ADLs, thereby improving the quality of life for both patient and caregiver. Research is ongoing, and it is estimated by the Alzheimer's Association that finding a treatment that could delay onset by only 5 years could reduce the number of individuals with AD by nearly 50% over the next 50 years (Alzheimer's Association, 2007). Although pharmacotherapy is not yet a cure, it does remain an important part of a total approach to caring for patients and families affected by AD.
References
Deborah Downey, MN RN CNRN CNS ANP, a nurse practitioner in neurology service at the Cleveland VA Medical Center, Cleveland, OH.
J Neurosci Nurs. 2008;40(1):55-59.
Pharmacology Update: Pharmacologic Management of Alzheimer Disease
Deborah Downey, MN RN CNRN CNS ANP
Published: 06/03/2008
Overview
Dementia is a major public health problem now estimated to affect about 5.1 million older adults in the United States and about 15 million worldwide. It is expected that there will be 13 million patients diagnosed with Alzheimer disease (AD) by the year 2050. The Alzheimer's Association anticipates that about 50% of adults older than 85 years of age will have a memory disorder, the largest percentage of those having AD (Alzheimer's Association, 2007). As the number of adults with memory disorders grows, so does the burden of caring for them, in terms of both the dollars spent on nursing facilities and the physical and psychological toll on family caregivers while the patient is still at home. This article reviews the pathophysiology of AD and describes the drug treatment options that are currently available. It is important to note that these drugs are not a cure but are aimed at slowing functional decline in AD patients.
Pathophysiology
Alzheimer disease is a progressive decline in the ability to perform routine activities of daily living (ADLs). Two primary pathologic processes are involved. In the first pathologic process, amyloid plaques, consisting of a sticky protein material, accumulate between cells in the brain and slow cellular transmission. These plaques are found mostly in the hippocampus, the area of the brain involved in formation of memories. The second pathologic process involves neurofibrillary tangles, intracellular collections of twisted protein filaments that destroy cells from within. Neurofibrillary tangles are present mostly in the cortex and hippocampus. These two pathologic processes pair together to wreak havoc within the nervous system, slowing transmission and impairing formation of new memories. The two processes may also be seen in other dementias, such as progressive supranuclear palsy, but are classically paired in AD (Geldmacher, 2001).
In addition to structural changes, alterations also occur in the neurotransmitters themselves, which facilitate neuron-to-neuron transmission. Acetylcholine (ACh), an important neurotransmitter involved in memory formation, is reduced as a result of cellular loss in the basal forebrain where most of the ACh is produced. There is also decreased production of norepinephrine (NE) and serotonin (5-HT), both of which contribute to the behavioral and cognitive changes associated with AD (Desai & Grossberg, 2005).
Diagnosis
Diagnosis of AD is made largely by history but must include loss of memory and one or more of the following: aphasia, apraxia, agnosia, and disturbance in executive functioning (American Psychiatric Association, 1994, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [DSM-IV]). Aphasia is an acquired disturbance of language; apraxia is the inability to order routine motor tasks, such as hair combing or dressing, into an organized way to complete them. Agnosia is the inability to interpret sensory information despite not having a primary sensory deficit. Executive functioning includes tasks such as judgment, complex reasoning, organization of tasks, and insight. In addition, there must be significant impairment in social or occupational function or a significant change from baseline abilities, and these changes must not occur during delirium or in the context of other medical disorders (American Psychiatric Association).
It is important to rule out reversible causes of dementia before making a definitive diagnosis of AD. Misdiagnosis deprives the patient of treatment for what may be a reversible condition. Practice parameters from the American Academy of Neurology (AAN) for diagnosis include testing for B12 deficiency and hypothyroidism, as these are common problems and represent potentially reversible causes of memory impairment in the elderly. Laboratory testing should include B12, folate, and thyroid stimulating hormone assay and testing for metabolic disturbances, including diabetes and electrolyte abnormalities. Although many clinicians still test for prior syphilis exposure, unless the patient has specific risk factors, the AAN does not recommend this as part of routine testing at this time (Knopman et al., 2001).
Regarding structural neuroimaging, AAN does not recommend either computed tomography (CT) or magnetic resonance imaging (MRI) as part of the initial evaluation; however, most clinicians are still obtaining some form of imaging to rule out a structural cause for memory impairment. Volumetric CT, positron emission tomography (PET), or single photon emission computed tomography (SPECT) also are not recommended for routine use as part of the initial evaluation; however, they may be appropriate in individual circumstances. It is important to note that the definitive diagnosis of AD is made only on postmortem tissue examination, but about 85% of those who meet current diagnostic criteria during their lifetime will meet neuropathologic criteria at autopsy (Knopman et al., 2001).
It is also important for the patient and family to determine whether the patient has another of the progressive dementias because the course can be quite different from AD, and making this determination offers the family the ability to plan for future care needs. Table 1 lists other progressive dementias that should be considered.
Table 1. Dementias
Risk factors for AD include age, family history of dementia, lower socioeconomic status, lower educational level, depression or other psychiatric illness, previous head injury, and alcohol abuse. Chronic medical problems such as diabetes, hypertension, ischemic coronary disease, and cerebral disease may worsen an existing memory impairment as well.
Treatment
In treating AD, it is important to recognize that it is a progressive disease. Patients will continue to decline functionally, whether they are treated or untreated. Stopping the functional decline is not a treatment goal because it is now known that neuronal loss has most likely been ongoing for some years before actual recognition of a memory disorder. Symptomatic treatment is aimed at improvement in function. A delay in decline does not necessarily mean neuronal loss is being stopped; treatment is aimed at preserving and facilitating remaining synaptic function, allowing a higher functional level for a longer time. When therapy is discontinued, it can be expected that decline will occur at the same rate as untreated dementia, as the rate of neuronal death is not affected (Geldmacher, 2001). Slowing decline can result in benefit for the patient and caregiver even if it does not manifest as clinical improvement.
Despite the many advances in pharmacologic therapy for neurological disease, 6 years after the Decade of the Brain, there are still only five drugs approved for dementia, although many more are in the research pipeline. The current drugs fall into two classes. The drugs in the acetylcholinesterase class are tacrine (Cognex), donepezil (Aricept), rivastigmine (Exelon), and galantamine (Razadyne). Tacrine, the first drug in this class, caused alterations in liver transaminases and liver failure and, although still available, is not a first-line therapy because there are now other safer choices (Tacrine will not be discussed in this article). Memantine (Namenda), an N-methyl-D-aspartate (NMDA) receptor antagonist, is in a separate class.
An important treatment paradigm for AD is the cholinergic benefit. If loss of ACh is the primary neurotransmitter defect, then increasing production, inhibiting destruction, or activating receptors all should translate into effective therapy (i.e., slow functional decline). ACh is broken down in the synaptic junction by acetylcholinesterase. By inhibiting this breakdown, drugs in the acetylcholinesterase group (donepezil, galantamine, and rivastigmine) prevent destruction of ACh.
In clinical trials for the acetylcholinesterase inhibitors, the cognitive portion of the Alzheimer Disease Assessment Scale (ADAS-Cog) was used to assess cognitive function. With some variability among the trials, the acetylcholinesterase inhibitors improved patients' ADAS-Cog score as compared to placebo. Secondary trial measures included evaluation of patients' abilities to maintain ADLs. Although most clinicians feel it is probably true that patients maintain ADL ability longer, definitive trial evidence of this benefit has yet to emerge. In addition, improvement in behavioral function has been reported, but it is more likely that the slowing of cognitive dysfunction delays the emergence of behavioral disturbance (Cummings, 2004).
Adverse effects of these drugs are related to their cholinergic properties. Nausea, gastrointestinal (GI) upset, diarrhea, weight loss, bradycardia, syncope, and insomnia can occur. Some patients complain of vivid dreams or nightmares; these symptoms improve with a decrease in dose. Although the drugs have similar profiles, if a patient has difficulty with side effects from one, clinicians sometimes find that another drug in the same class will be better tolerated; however, clinical data to support this observation are lacking. Anticholinergic drugs may inhibit the effect of all the drugs in this class ( Table 2 ).
Table 2. Treatment Options
Donepezil (Aricept) is usually started at 5 mg daily for 1 month and then increased to 10 mg daily. The once-daily dosing is advantageous for patients who live alone or who have difficulty managing medications. Although the package insert advises titration after 1 month, most patients will tolerate a 2-week titration schedule. This drug is metabolized within a minor substrate of the CYP2D6 and 3A4 metabolic system; competing drugs make the drug less available for its therapeutic effect. St. John's Wort may also decrease the drug effect of donepezil. As with all the drugs in the acetylcholinesterase class, GI side effects are the most common; however, slowing the taper up to 10 mg usually improves any adverse GI effects. Donepezil use also appears to cause a higher incidence of vivid dreaming than other drugs in this class, but switching to a morning dose improves sleep quality. Donepezil is now available in an orally disintegrating form as well.
Galantamine (Razadyne) is started at 4 mg bid and is increased to 8 mg and then 12 mg bid at 4-week intervals. Galantamine has recently been approved for a once-daily sustained-release form, and this is now available. This drug also is metabolized within a minor substrate of the CYP2D6 and 3A4 systems, and St. John's Wort may decrease blood levels of donepezil. The name of this drug has recently been changed to Razadyne because its former name, Reminyl, was too similar to that of another drug and was considered to be potentially confusing.
Rivastigmine (Exelon) dosing starts at 1.5 mg bid and is increased to 3 mg bid, then 4.5 mg bid, and then 6 mg bid at 4-week intervals. Rivastigmine has a nonhepatic metabolism, unlike donepezil and galantamine, which are metabolized through the CYP2D6 and CYP3A4 pathways. However, it has been associated with esophageal irritation and should be used with caution in patients with peptic ulcer disease or those using nonsteroidal antiinflammatory drugs. As is the case with the other drugs in this class, cholinergic drugs may potentiate the drug effect, while anticholinergic drugs may inhibit effectiveness. Both rivastigmine and galantamine are available in liquid forms for those patients with swallowing difficulties; no intravenous formulations are available.
One caveat with all the anticholinesterase drugs is that if they are stopped for any reason, they must be restarted at the lowest dose and titrated up again as in the original taper schedules. There have been case reports of esophageal rupture when rivastigmine was stopped and then restarted at the highest tolerated dose.
Memantine (Namenda) is a recently approved NMDA antagonist that is thought to interfere with glutaminergic overstimulation (excitotoxicity). By interfering with excitotoxicity, memantine affects the NMDA receptors implicated in memory processing and the pathology of AD (Reisberg et al., 2003). Clinical trials looked at memantine alone, memantine with placebo, and memantine with donepezil, a cholinesterase inhibitor. The regimens of memantine alone and memantine with donepezil were found to be effective across the domains of cognition, function, behavior, and global status, and the effect was statistically significant (Reisberg et al.). Memantine is currently approved by the U.S. Food and Drug Administration for use in patients with moderate to severe AD. The dosing schedule is 5 mg daily for 1 week, 5 mg bid for 1 week, 10 mg in the morning and 5 mg in the evening for 1 week, and then 10 mg bid. Adverse effects include dizziness, headache, GI upset, and hypertension. There have been case reports of worsening behavioral status and agitation, which have cleared when stopping the drug. Caution also needs to be used when memantine is taken with other NMDA antagonists such as amantadine or dextromethorphan. Memantine undergoes nonhepatic metabolism and is excreted by the kidney, so caution should be taken by patients with renal insufficiency or when other renally excreted drugs are used. Dosages may need to be adjusted or lowered if creatinine rises; sometimes the drug will need to be stopped altogether. Memantine can be taken without regard to meals.
Other Treatment Options
Antioxidant Therapy
Antioxidant therapy has also been studied for AD. In 2001, the AAN Practice Parameter: Management of Dementia (an evidence-based review) suggested adding 1,000 units of vitamin E twice daily as a practice guideline, not a standard (Doody et al., 2001). Despite great hopes, more recent studies have shown that vitamin E has little benefit in influencing the rate of progression from mild cognitive impairment to AD as compared to placebo (Petersen et al., 2005). Less clear is the issue of whether vitamin E slows progression of already diagnosed dementia. Although many clinicians still use vitamin E as additive therapy for patients with AD, its effectiveness remains unclear. A recent study by the Alzheimer's Disease Cooperative Study Group showed that vitamin E changed the likelihood of progressing from mild cognitive impairment to AD at the end of 3 years but that patients on donepezil had a lower rate of progression to AD for the first 12 months. After that, the rate of progression was similar to that of vitamin E. Although 12 months does not seem like a significant amount of time, the impact of that time span on caregivers can be significant from both a caregiving and a financial standpoint by delaying placement in a care facility (Petersen et al.).
Hormone Replacement Therapy
Previous studies and epidemiological reports have suggested that hormone replacement therapy (HRT) might be effective in reducing the onset of cognitive decline. However, the recently published results of the Women's Health Initiative clearly showed an increased risk of dementia among postmenopausal women who had not had any cognitive deficit at enrollment and who were assigned to the treatment group and thus received estrogen (Shumaker et al., 2003). As a result, taking HRT for the prevention of dementia is not currently recommended.
Antiinflammatory Agents
Pathology reviews of brains of patients with AD have shown microscopic inflammation. This finding has led to numerous studies of antiinflammatory agents for AD, including diclofenac, rofecoxib, naproxen, and, most recently, ibuprofen. There is insufficient evidence at this time to support using these drugs as protective agents for AD (Cummings, 2004).
Herbal Supplements
Herbal supplements are often used by patients in conjunction with other prescribed therapies. A frequent choice is ginkgo biloba, whose pharmacologic effect is primarily as a flavoglycoside, a potent free-radical scavenger. It may also function as an antioxidant. A formal review of more than 50 articles in which ginkgo biloba was used for supporting cognitive function showed that on a quantitative analysis of the literature, there was a small but significant effect after 3 to 6 months' treatment with 120?240 mg of ginkgo, but the data were inconsistent (Oken, Storzbach, & Kaye, 1998). There is currently an ongoing primary prevention trial using ginkgo that is studying the rate of development of AD. This may be limited, however, by the possibility of bleeding, as in vitro studies show evidence that ginkgo inhibits platelet aggregation (Basch & Ulbicht, 2005).
Summary
Although the diagnosis of AD can be devastating, treatment options exist that can slow the disease's progression and allow patients to continue performing ADLs, thereby improving the quality of life for both patient and caregiver. Research is ongoing, and it is estimated by the Alzheimer's Association that finding a treatment that could delay onset by only 5 years could reduce the number of individuals with AD by nearly 50% over the next 50 years (Alzheimer's Association, 2007). Although pharmacotherapy is not yet a cure, it does remain an important part of a total approach to caring for patients and families affected by AD.
References
- Alzheimer's Association. (2007). Alzheimer's disease facts and figures 2007. Retrieved December 4, 2007, from www.alz.org/national/documents/report_alzfactsfigures2007.pdf.
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author.
- Basch, E. M., & Ulbicht, C. E. (2005). Natural standard herb and supplement handbook. St. Louis: Elsevier Mosby.
- Cummings, J. (2004). Alzheimer's disease. New England Journal of Medicine, 351, 56?67.
- Desai, A., & Grossberg, G. (2005). Diagnosis and treatment of Alzheimer's disease. Neurology,64(Suppl. 3), S34?S39.
- Doody, R., Stevens, J., Beck, C., Dubinsky, R., Kaye, J., Gwyther, L., et al. (2001). Practice parameter: Management of dementia (an evidence-based review). Neurology, 56, 1154?1166.
- Geldmacher, D. (2001). Contemporary diagnosis and management of Alzheimer's dementia. Newton, PA: Handbooks in Health Care Co.
- Knopman, D., DeKosky, S., Cummings, J., Chui, H., Corey-Bloom, J., Relkin, N., et al. (2001). Practice parameter: Diagnosis of dementia (an evidence-based review). Neurology, 56, 1143?1153.
- Oken, B., Storzbach, D., & Kaye, J. (1998). The efficacy of ginkgo biloba on cognitive function in Alzheimer disease. Archives of Neurology, 55, 1409?1415.
- Petersen, R., Thomas, R., Grundman, M., Bennett, D., Doody, R., Ferris, S., et al., for the Alzheimer's Disease Cooperative Study Group (2005). Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine,352, 2379?2388.
- Reisberg, B., Doody, R., Stoffler, A., Schmitt, F., Ferris, S., & Mobius, H. J. (2003). Memantine in moderate-to-severe Alzheimer's disease. New England Journal of Medicine, 348, 1333?1341.
- Shumaker, S. A., Legault, C., Rapp, S. R., Thal, L., Wallace, R. B., Ockene, J. K., et al. (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative Memory Study: A randomized controlled trial. Journal of the American Medical Association,289, 2651?2662.
Deborah Downey, MN RN CNRN CNS ANP, a nurse practitioner in neurology service at the Cleveland VA Medical Center, Cleveland, OH.
J Neurosci Nurs. 2008;40(1):55-59.