Auvelity, the New Fast-Acting Pill for Depression
"In August 2022, the FDA approved Auvelity (dextromethorphan/bupropion) to treat depression in adults. It’s an oral medication that works differently than other antidepressants."
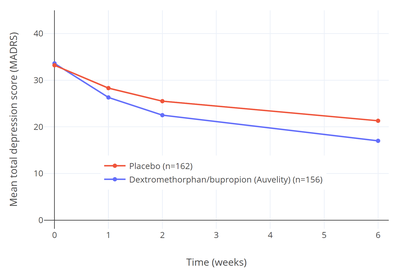
"Most oral antidepressants need about 4 to 8 weeks (1 to 2 months) before their full benefits kick in. Auvelity appears to be effective after 1 week."